Abstract
Background Vincristine (VCR)-steroid pulses have been a component of the maintenance phase of therapy for pediatric acute lymphoblastic leukemia (ALL) since the 1970s. However, major cooperative groups differ in how these are incorporated, if at all. Two previous systematic reviews (Richards 1996, Yetgin 2010) examining this question supported the inclusion of VCR/Steroid pulses. Yet, two recent randomized control trials (RCTs; Angiolillo 2021, Yang 2021) demonstrated no impact on event-free survival (EFS) with decreased frequency of maintenance pulses.
Objectives The objectives of this systematic review and meta-analysis were to examine the impact of reducing frequency of VCR/steroid pulses in maintenance therapy for newly diagnosed pediatric patients with B-cell ALL overall, and according to era of therapy (historical versus contemporary).
Methods We searched MEDLINE, EMBASE, and Cochrane CENTRAL for RCTs and meta-analyses of RCTs from inception until February 2022. Additional studies were identified from clinical trials databases, grey literature, and screening reference lists of previous systematic reviews. Two authors reviewed all studies and included those which addressed newly diagnosed pediatric patients with B-cell ALL, where randomization examined a change in how VCR/steroid pulses were provided in maintenance, and provided at minimum EFS outcomes. We excluded trials which compared VCR/steroid pulses to more intensive maintenance therapies with additional chemotherapy agents.
Study data were extracted and risk of bias assessed using Cochrane Risk of Bias 2.0 tool by two authors. Hazard (HR) and odds ratios (OR) with associated 95% Confidence Intervals (CI) were calculated for EFS, overall survival (OS), incidence of relapse, and adverse events, including a composite measure of non-hepatic toxicities. We created historical and contemporary subgroups; the latter were defined as treatment protocols that: 1) provided a delayed intensification or re-intensification phase based on Protocol III from early Berlin-Frankfurt-Munster (BFM) trials, and; 2) avoided prophylactic cranial radiation in all but the highest risk patients. A random effects meta-analysis was completed using the generic inverse variance method for time-to-event data and the Mantel-Haenszel method for dichotomous events. Heterogeneity was assessed using I2 statistics.
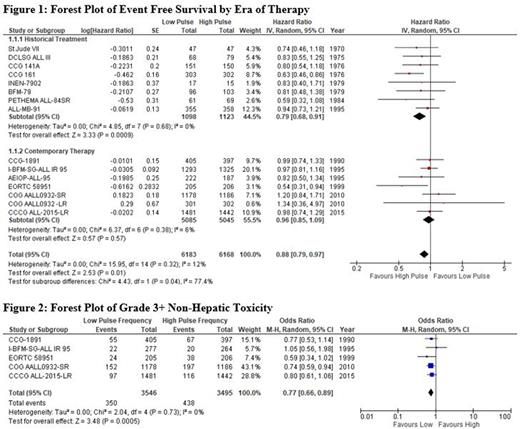
Results Our systematic review included 25 publications representing 16 completed and 2 ongoing RCTs, with a total of 12,513 patients (low pulse frequency, 6,269 and high pulse frequency, 6,244). No study was at low risk of bias; however, trials in the contemporary sub-group were more robust, with only minor concerns identified mainly due to the lack of blinding.
Meta-analysis of EFS data from 14 trials (Figure 1) suggested no benefit between high or low pulse frequency in contemporary trials (HR 0.96, 95% CI 0.85-1.09, p=0.57), while in historical trials high pulse frequency was favored (HR 0.79, 95%CI 0.68-0.91, p<0.001). The difference between these two subgroups was statistically significant (p= 0.04). Similar findings were found in our meta-analysis of overall relapse risk, with no benefit seen for higher pulse frequency in contemporary trials (OR 1.02, 95%CI 0.83-1.25, p=0.85) but seen in historical trials (OR 0.68, 95%CI 0.47-0.99, p=0.05; p=0.07 for difference between groups). We found no significant impact of reduced pulse frequency on OS or isolated central nervous system (CNS) relapse in either subgroup. In 5 trials with toxicity data, high frequency pulses were associated with increased risk of Grade 3+ non-hepatic events (OR 1.31, 95%CI 1.12-1.52; p<0.001, Figure 2).
Conclusions This systematic review suggests that the previous benefit conferred by frequent pulses of VCR/steroids in maintenance therapy for pediatric B-cell ALL in historical trials no longer significantly improves outcomes in contemporary protocols, but is associated with increased toxicity. These results will help guide development of the next phase of clinical trials in the field of pediatric ALL.
Disclosures
Gupta:Jazz Pharmaceuticals: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal